It is known that intracellular glutathione (GSH) interacts with compounds containing a disulfide bond, and as a result of the disulfide exchange reaction it is converted to a dimeric form (GSSG).
To study this reaction, which takes place in the intracellular environment, the interaction of the disulfide derivative of DPBPI with the reduced form of glutathione was realized.
Cystine-DPBPI (5) was dissolved simultaneously with the reduced form of glutathione in a 5% aqueous solution of dimethylsulfoxide (DMSO). The course of the reaction was monitored by the change in the chromatographic mobility of the reaction product compared to the initial cystine-DPBPI (4). (Figure 2)
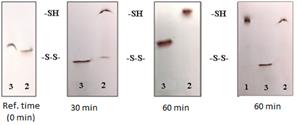
Fig. 2 – Interaction of dimer (5) with glutathione (1 – monomer standart, 2 – disulfide in the presence of glutathione, 3 – disulfide standart).
Analysis of the data obtained suggests that the dimer (5), when interacting with glutathione, converts to the monomer (4), which apparently occurs during internalization of substances with a disulfide bond inside into the cell.
Such interaction can lead to inactivation of the glutathione antioxidant system (due to the cystine-induced transition of the reduced form of glutathione to the oxidized one), weakening of the antioxidant system of tumor cells, and, as a result, to an increase of the photodynamic effect.
Conclusions
In the present work, bacteriochlorophyll a derivatives with the residues of four sulfur-containing amino acids were obtained for the first time. It has been found that the oxidation of free thiol groups in cysteine-containing bacteriopurpurinimide occured under the action of atmospheric oxygen with disulfide formation, which undergoes monomerization with the participation of the reduced form of glutathione in the cell. In the case of oxidation of the methionine residue in the conjugate with dipropoxybacteriopurpurinimide, a stable sulfoxide is formed.
The mechanism of suppression of the antioxidant system of tumor cells under the action of sulfur-containing bacteriochlorophyll a derivatives is proposed.
Acknowledgements.
This work is supported by the Russian Science Foundation (RSF Grant: 16-13-10092), as well as the Russian Foundation for Basic Research (Grant RFBR: No. 16-03-00519).
References
1. Aruoma O.K., Halliwell B., Hoey B.M., Butler J. Free Radical Biology & Medicine 1989, 6, 593-597.
2. Qiu J., Cheng R., Zhang J., Sun H., Deng Ch., Meng F. and Zhong Zh. Biomacromolecules 2017, 18, 3207-3214.
3. Azzouzi A.R., Barret E., Bennet J., Moore C., Taneja S., Muir G., Villers A., Coleman J., Allen C., Scherz A., Emberton M. World Journal Of Urology 2015, 33, 945-953.
4. Bak D.W., Weerapana E. Mol. BioSyst. 2015, 11, 678-697.
5. Grin M.A., Mironov A.F. Synthetic and Natural Bacteriochlorins: Synthesis, Properties and Applications. In: Chemical Processes with Participation of Biological and Related Compounds. Biophysical and Chemical Aspects of Porphyrins, Pigments, Drugs, Biodegradable Polymers and Nanofibers (Lomova T.N., Zaikov G.E., Eds.). Brill, Leiden-Boston, 2008, p. 5–43.
6. Mironov A.F. Russian Chemical Bulletin 2016, 65, 1-17.
7. Pandey R. K., Goswami L. N., Chen Y., Gryshuk A., Missert J. R., Oseroff, A., Dougherty T. J. Lasers Surg. Med. 2006, 38, 445−467.
8. Kessel D., Wooburn K., Gomer C.J., Jagerovic N., Smith K. M. J. Photochem. Photobiol. B: Biology 1995, 28, 13-18.
9. Taber S.W., Fingar V.H., Coots C.T., Wieman T.J. Photodynamic therapy using mono-L-aspartyl chlorine // Clin. Cancer. Res. 1998, 4, 2741- 2746
10. Mody T.D. Pharmaceutical development and medical applications of porphyrin-type macrocycles // J. Porphyrins and Phthalocyanines 2000, 4, 362-367.
11. Pandey R.K. Recent advances in photodynamic therapy // J. Porphyrins and Phthalocyanines 2000, 4, 368-373.
12. MacDonald I., Dougherty T.J. J. Porphyrins and Phthalocyanines 2001, 5, 105-129.
13. Spikes J.D., Boomer J.C. Chlorophyll and related pigments as photosensitizers in biology and medicine. In: Chlorophylls, Scheer H. Ed. CRC Press: Boston. 1996, 1182-1204.
14. Guo X., Wang L., Wang Sh., Li Y., Zhang F., Song B., Zhao W. Bioorganic & Medicinal Chemistry 2015, 25, 4078-4081
15. Pantiushenko I.V., Rudakovskaya P.G., Starovoytova A.V., Mikhaylovskaya A.A., Abakumov M.A., Kaplan M.A., Tsygankov A. A., Majouga A.G., Grin M.A., Mironov A.F. Biochemistry 2015, 80, 752-762
16. Besouw M., Masereeuw R., den Heuvel L., Levtchenko E. Drug Discovery Today 2013, 18, 785–792.
17. Mironov A.F., Grin M.A., Pantushenko I.V., Ostroverkhov P.V., Ivanenkov Y.A., Filkov G.I., Plotnikova E.A., Karmakova T.A., Starovoitova A.V., Burmistrova N.V., Yuzhakov V.V., Romanko Y.S., Abakumov M.A., Ignatova A.A., Feofanov A.V., Kaplan M.A., Yakubovskaya R.I., Tsigankov A.A., Majouga A.G. Journal of Medicinal Chemistry 2017, 60, 10220-10230.
18. Yuzhakov V.V., Burmistrova N.V., Fomina N.K., Bandurko L.N., Sevanjkaeva L.E., Starovoytova A.V., Yakovleva N.D., Tsyganova M.G., Ingelj I.E., Ostroverkhov P.V., Kaplan M.A., Grin M.A., Mazhuga A.G., Mironov A.F., Galkin V.N., Romanko Y.S. Biomedical Photonics 2016, 5, 4-14.
19. Ramanathan, B., Jan, K.-Y., Chen, C.-H. et al. Cancer Res. 2005, 65, 8455–8469.
20. Attia, S., Kolesar, J., Mahoney, M.R. et al. Invest. New Drugs 2008, 26, 369–379.
21. Hoffman R.M. Biochim Biophys Acta 1984, 738, 49–87.
22. Cavuoto P., Fenech M.F. Cancer Treatment Reviews 2012, 38, 726-736.
23. Chissov V.I., Yakubovskaya R.I., Mironov A.F., Grin M.A., Plotnikova E.A., Morozova N.B., Tsigankov A.A. 2014, “Preparation for photodynamic therapy and method for photodynamic therapy of cancer with using it” RU2521327 C1
24. Pantushenko I.V., Grin M.A., Yakubovskaya R.I., Plotnikova E.A., Morozova N.B., Tsigankov A.A., Mironov A.F. Fine Chemical Technologies 2014, 9, 3-10
25. Weissbacha H., Resnicka L., Brotb N. Biochimica et Biophysica Acta 2005, 1703, 203–212.
26. Lee B.C., Gladyshev V.N. Free Radical Biology & Medicine 2011, 50, 221-227.
Received date
Accepted date