Synthesis of Cys-DPBPI (4).
DPBPI (3) (15 mg, 21.5 μmol) and EEDQ (8 mg, 32.3 μmol) were dissolved in CH2Cl2 (1 ml) with stirring. After 15 minutes, cysteine methyl ester hydrochloride (7.38 mg, 43.0 μmol) was added to the reaction mass. The reaction was carried out for 24 hours under argon atmosphere, in the dark with stirring. The product (4) was isolated by extraction with DCM (3x50 ml) and with water (500 ml), dried with Na2SO4 and purified using preparative TLC (CH2Cl2 / CH3OH, 45/1, v / v). The yield of the desired compound (4) was 5,7 mg (38%).
UV/VIS, λ max, nm: (relative intensities of peaks): 368, 420, 544, 798 (1: 0.51: 0.39: 0.43).
MALDI MS: m/z calculated for C43H56N7O7S [М+H]: 814,01, found.: 814,25[М+H].
1H NMR: (300 MHz, DMSO – d6) δ, p.p.m: 8.77 (Н, s, 5-Н), 8.68 (Н, s, 10-Н), 8.64 (Н, s, 20-Н), 8.15 (H, d, 173-NH), 7.13 (H, m, SH), 5.30 (Н, m, 17-Н), 4.33 (4H, m, -O CH2 CH2CH3), 4.20 (2Н, m, 7-Н, 18-Н), 4.09 (Н, m, 8-H), 3.98 (3Н, s, 12-CH3), 3.56 (4H, m, СHCH2SH), 3.34 (3Н, s, 2-CH3), 2.70 (3Н, s, 32-CH3), 2.56 (Н, m, 172-CH2), 2.45 (3Н, m, 81-СН2, 171-СН2, 172-CH2), 2.15 (2Н, m, 81-СН2, 171-СН2), 1.91 (3H, J=7.24 Hz d, 7-СН3), 1.62 (9Н, m, 18-СН3, -OCH2CH2 CH3), 1.25 (4H, m, -OCH2 CH2 CH3), 1.24 (3Н, m, 82-CH3), 0.11 (s, NH), -0.22 (s, NH).
Synthesis of Cys-Cys-DPBPI (5).
DPBPI (3) (15 mg, 21.5 μmol) and EEDQ (8 mg, 32.3 μmol) were dissolved in CH2Cl2 (1 ml) with stirring. After 15 minutes, cystine methyl ester hydrochloride (22.01 mg, 64.5 μmol) was added to the reaction mass. The reaction was carried out for 24 hours under argon atmosphere, in the dark with stirring. The product (5) was isolated by extraction with DCM (3x50 ml) and with water (500 ml), dried with Na2SO4 and purified using preparative TLC (CH2Cl2/CH3OH, 35/1, v / v). The yield of the desired compound (5) was 9,9 mg (66%).
UV/VIS, λ max, nm: (relative intensities of peaks): 368, 420, 544, 798 (1: 0.50: 0.37: 0.44).
MALDI MS: m/z calculated for C86H108N14O14S2 [M+H]: 1625.99, found: 1626.47 [М+H].
1H NMR (300 MHz, CDСl3) δ, p.p.m: 8.63 (s, H, 10-H), 8.56 (s, H, 5-H), 8.38 (s, H, 20-H), 7.26 (d, H, 173-NH), 4.61 (m, O-CH2-) 4.25 (m, 18-H, 7-H), 4.12 (m, 8-H), 3.68 (s, 12-CH3), 3.53-3.46 (m, 4Н, –CHСН2S-), 3.50 (s, 175-CH3), 3.53 (s, 2-CH3), 3,27 (s, 32-CH3), 2.77 (s,177 -OMe), 2.75 (m, 81-CH2), 2.73-2.66 (m, 177-CH2S-), 2.40 (m, 171-H, 81-H), 1.83 (d, J=7.31, 7-CH3), 1.70 (m, 18-CH3), 1.12 (3H, m, 82–CH3), 0.13 (s, NH), –0.18 (s, NH).
Synthesis of Met-DPBPI (6).
DPBPI (3) (30 mg, 43 μmol) and EEDQ (16 mg, 64.6 μmol) were dissolved in CH2Cl2 (1 mL) with stirring. After 15 minutes, methionine methyl ester hydrochloride (17.2 mg, 86 μmol) was added to the reaction mass. The reaction was carried out for 24 hours under argon atmosphere, in the dark with stirring. The product (6) was isolated by extraction with DCM (3x50 ml) and with water (500 ml), dried with Na2SO4 and purified using preparative TLC (CH2Cl2/CH3OH, 45/1, v / v). The yield of the desired compound (6) was 16,8 mg (56%).
UV/VIS, λ max, nm: (relative intensities of peaks): 368, 420, 544, 798 (1: 0.48: 0.38: 0.45).
MALDI MS: m/z calculated for C45H59N7O7S [M+H]: 842.06, found: 842.38 [М+H].
1H NMR (300 MHz, CDСl3) δ, p.p.m: 8.62 (s, H, 5-H), 8.55 (s, H, 10-H), 8.38 (s, H, 20-H), 7.05 (d, H, 173-NH), 5.14 (m, H, 17-Н), 4.78 (m, H, - CH CH2CH2SCH3), 4.56 (m, 4H, -OCH2 CH2 CH3), 4.22 (m, 2H, 18-H, 7-H), 4.01 (m, H, 8-H), 3.70 (s, 3H, -OCH3 Met), 3.63 (s, 3H, 12-CH3), 3.29 (s, 3H, 2-CH3), 2.74 (s, 3Н, 32-CH3), 2.67 (m, Н, 172-CH2), 2.58 (m, 2Н, -CHCH2 CH2 SCH3), 2.38 (m, 3H, 81-СН2, 171-СН2, 172-CH2), 2.18 (m, 2Н, -CH CH2 CH2SCH3), 2.10 (s, 3Н, -S-CH3), 2.04 (m, 2Н,, 81-СН2, 171-СН2), 1.68 (d, J=7.19 3H, 7-CH3), 1.61 (м, 9H, -OCH2CH2 CH3, 18-CH3), 1.12 (m, 3Н, 82-CH3), 0.15 (s, Н, NH), –0.15 (s, Н, NH).
Synthesis of Met(SO)-DPBPI (7).
To the DPBPI conjugate with methionine methyl ester (6) (10 mg, 11.87 μmol) 30% aq. solution of H2O2 (0.5 ml) was added. The reaction proceeded in the dark for 30 minutes. The product (7) was isolated by extraction with DCM (3x50 ml) with water (500 ml), dried on Na2SO4. Compound was purified by preparative TLC (CH2Cl2/CH3OH, 30/1, v / v). The yield of the compound (7) was 8,5 mg (85%).
UV/VIS, λ max, nm: (relative intensities of peaks): 368, 420, 544, 798 (1: 0.49: 0.37: 0.46).
MALDI MS: m/z calculated for C45H59N7O8S [M+H]: 858,06, found: 858,72 [М+H].
1H NMR (300 MHz, CDСl3) δ, p.p.m: 8.62 (s, 5-H), 8.55 (s, 10-H), 8.38 (s, H, 20-H), 7.23 (d, 174-NH), 5.14 (m, 1H, 17-Н), 4.78 (m, H, - CH CH2CH2SCH3), 4.56 (m, 4H, -OCH2CH2CH3), 4.22 (m, 2H, 18-H, 7-H), 4.01 (m, 1H, 8-H), 3.70 (s, 3H, -OCH3 Met), 3.63 (s, 3H, 12-CH3), 3.29 (s, 3H, 2-CH3), 2.74 (s, 3Н, 32-CH3), 2.67 (m, 1Н, 172-CH2), 2.58 (m, 2Н, -CHCH2 CH2 S(O)CH3), 2.38 (m, 3H, 81-СН2, 171-СН2, 172-CH2), 2.33 (m, 2Н, -CH CH2 CH2SCH3), 2.18 (s, 3Н, -S-CH3), 2.04 (m, 2Н,, 81-СН2, 171-СН2), 1.68 (d, J=7.26 3H, 7-CH3), 1.61 (m, 9H, -OCH2CH2CH3, 18-CH3), 1.12 (m, 3Н, 82-CH3), 0.12 (s, 1Н, NH), –0.12 (s, 1Н, NH).
Results and Discussion
As a starting compound, natural bacteriochlorophyll a (Bcl a) (1) was used which was obtained from the Rhodobacter capsulatus bacteria biomass by extraction with isopropyl alcohol. The long-wavelength maximum of the pigment absorption is in the region of 770 nm. Synthesis of bacteriopurpurin (2) - a bacteriochlorophyll a derivative containing an anhydride exocycle - was carried out by oxidizing the latter with air oxygen under alkaline conditions. The reaction was monitored spectrophotometrically from the shift of the absorption band to the 812 nm region. The resulting bacteriopurpurin is a labile compound because of the presence of an anhydride cycle, ready to the opening in alkaline medium. Therefore, O-propyloxime-N-propoxybacteriopurpurinimide (DPBPI) (3), a promising photosensitizer of high stability, having an absorption band at 800 nm and showing significant photoinduced activity on tumor cells and on tumors of various genes in animals was proposed as a leading compound in this reasearch. [23,24] (Scheme 1)

Scheme 1. Synthesis of O-propyloxime-N-propoxybacteriopurpurinimide (DPBPI).
Based on DPBPI (3), its derivatives with methyl esters of cysteine (Cys-DPBPI, 4), cystine (Cys-Cys-DPBPI, 5), methionine (Met-DPBPI, 6) and methionine sulfoxide (MetSO-DPBPI, 7) were synthesized (Scheme 2).

Scheme 2. Derivatives of DPBPI with sulfur-containing amino acids.
The absorption spectra of all the sulfur-containing compounds obtained at the same concentration had an absorption band of Q2 of the same intensity, except for compound 5. (Fig. 1)

Fig. 1 –Absorption spectra of DPBPI and its sulfur-containing derivatives.
The preparation of the O-propyloxime-N-propoxybacteriopurpurinimide derivative with methyl ester of cysteine was carried out by creating an amide bond between the carboxyl group of the propionic residue at position 17 of the macrocycle and the α-amino group of cysteine in the presence of the condensing agent - N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ). The reaction progress was monitored chromatographically by increasing the mobility of the reaction product (4). The latter turned out to be unstable in the air and oxidized to a dimer (5), which was obtained by the on-going synthesis from DPBPI and methyl ester of cystine. The synthesis was carried out under conditions similar to those described earlier (Scheme 3). The compounds (4) and (5) obtained were characterized by mass- and NMR spectra.

Scheme 3. Synthesis of DPBPI derivatives with methyl esters of cysteine и cystine.
Another sulfur-containing amino acid, attached to the DPBPI and not containing the thiol group, was methionine (6). The latter did not dimerize like cysteine and its oxidation proceeded differently (Scheme 4).
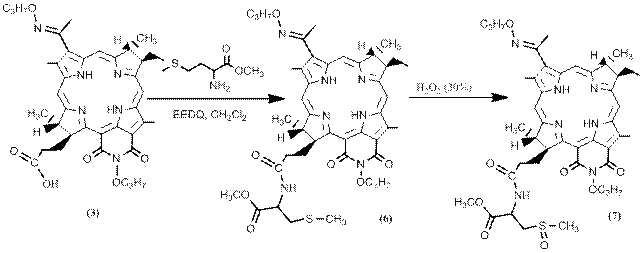
Scheme 4. Obtaining of DPBPI derivatives with methionine and methioninesulfoxide methyl esters.
It was shown that under the influence of atmospheric oxygen, the sulfur atom is slowly oxidized to form methionine sulfoxide (7). More easily, a similar conversion occurs under the action of a 30% solution of hydrogen peroxide. [25, 26]
The structure of the conjugate (6) was confirmed by the NMR spectrum, in which a signal of the methyl group is observed at the sulfur atom in the region of 2.1 ppm. In the NMR spectrum of the oxidized form (7), the signals from the amino acid are shifted to the weak-field region.
Thus, the oxidized forms of the amino acid derivatives of DPBPI, including conjugates with cystine and methionine sulfoxide, are more stable comparing to the original pigments and can be considered as prodrugs in the development of pharmacologically active substances.