Bohr model and its properties
Bohr model of the atom describes the atomic structure. Although Bohr model has been supplanted by other models, its general principles are still valid.
The system of an atom, consisting of nucleus and electrons, has some stationary states. In these states, the atom does not emit or absorb light. Each of these states has its own energy level. The atom absorbs energy while moving from a low energy level to higher levels, and emits it when moving in the opposite direction. The emitted or absorbed energy is equal to the energy difference of the stationary states between which the transition takes place:
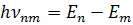
where
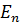 and
and 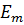 are the energies of the stationary states and
are the energies of the stationary states and
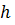 is Planck's constant
is Planck's constant
Then the radiation frequency is:
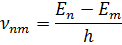
Atomic emission and absorption spectra
We now turn to the emission and absorption spectra of light. Like the unique fingerprints of people, the atoms of one chemical element emit a characteristic spectrum of light, peculiar only to them.
The individuality of the spectra is due to the fact that they contain only those frequencies at which the atoms of a given element emit or absorb. And the energies of the stationary states of the atoms of each chemical element are different. Therefore, the electron can move between certain states, radiating or absorbing certain amounts of energy.
Spectral analysis is based on the uniqueness of the spectra of various chemical elements. It is a method of determining the chemical composition of a substance by its ability to emit and absorb light.
Using spectral analysis, scientists research the chemical composition of various stars which led to the discovery of such chemical elements as rubidium, caesium and helium.
But the main contribution to the spectra of stars makes hydrogen. Its stationary levels, and hence the frequencies of emission and absorption, are well known and recognizable. Comparing them with the observed spectra, one can quite accurately calculate the radial velocity of the source.
Thermal radiation
Unlike the emission spectrum of rarefied gases, the emission spectrum of solids, liquids, and dense gases is continuous. This means that they emit at all possible frequencies. The fact is that the formation of an emission line-spectrum, described above, during the transitions of atoms between stationary states is valid only for a free atom.
However, if the atom constantly interacts with other atoms, the position of its energy levels will depend on this interaction. This phenomenon is called energy level splitting. The number of resulting levels will be huge because of the extremely large number of interacting atoms. As a result, all possible transitions between all possible levels give so many lines in the spectrum that they all merge into one continuous band.
To sum up, thermal radiation, generated by thermal motion particles in the star, is partially absorbed by photosphere, mainly consisting of hydrogen, in accordance with its absorption spectrum. The rest of the emitted frequencies reach the receiver to be examined and researched. The ways to infer the temperature from the received frequencies will be described in the next chapter.