Prelab conference. Topics for discussion:
1. Antigens, their nature, properties, application.
2. Bacterial antigens.
3. Antibodies (immunoglobulins). Structure and properties of immunoglobulins.
4. Classes of immunoglobulins, their characteristics.
5. Serological diagnostic tests based on the antigen-antibody interactions.
6. Agglutination test. Mechanism, components, application.
7. Indirect (passive) hemagglutination test (IHA-test).
8. Incomplete antibodies. Coomb’s test.
9. Precipitation test, mechanism, components, procedure, and application.
10. The components of serological reactions (diagnosticums, diagnostic antibody-containing antisera).
11. Pre-absorbed immune antisera. Preparation and application (Castellani technique).
¨ Antigens
Any substance that specifically stimulates an immune response when introduced into the body is an antigen or immunogen. Several characteristics of antigens are known.
1) Antigens are usually composed of protein or polysaccharide or contain these macromolecules as a major constituent. Lipids and nucleic acids are poor antigens unless linked to a protein or polysaccharide.
2) Some are soluble; others are particulate. Soluble bacterial exotoxins are antigenic. A bacterium may have several types of particulate antigens.
3) In general, larger molecules stimulate a more intense immune response than smaller molecules. Molecules below molecular weight 1000 are very poor antigens.
4) Antigens must be recognized by the host as foreign before they stimulate an immune response.
5) An antigen contains chemically distinct sites, called antigenic determinants (or epitopes), that define its specificity.
6) Small molecules that are nonantigenic by themselves may sometimes become antigenic determinants when coupled to large carrier molecules. These small compounds are called haptens. Haptens react with their specific antibodies, but unless they are attached to larger carrier molecules they are too small to stimulate the production of antibodies.
Thus, the features of antigens that determine immunogenicity in the immune response are as follows:
A. Foreignness: In general, molecules, recognized as «self» are not immunogenic; for immunogenicity, molecules must be recognized as «nonself».
B. Molecular Size: The most potent immunogens are usually large proteins. Generally, molecules with a molecular weight less than 10,000 are weakly immunogenic, and very small ones (e.g., amino acids) are non-immunogenic.
C. Chemical and Structural Complexity: A certain amount of chemical complexity is required. For example, amino acids homopolymers are less immunogenic than heteropolymers containing two or three different amino acids.
D.  Antigenic Determinants (Epitopes): The smallest unit of a complex antigen that is capable of binding to an antibody is known as an antigenic determinant, or epitope. An antigen can have one or more determinants. In general, a determinant is roughly five amino acids or sugars in size.
Antigenic Determinants (Epitopes): The smallest unit of a complex antigen that is capable of binding to an antibody is known as an antigenic determinant, or epitope. An antigen can have one or more determinants. In general, a determinant is roughly five amino acids or sugars in size.
E. Genetic Constitution of the Host: Two strains of the same species of animal may respond differently to the same antigen because of different composition of immune response genes.
F. Dosage, Route, and Timing of Antigen Administration: Since the degree of the immune response depends on the amount of antigen given, the immune response can be optimized by carefully defining the dosage (including the number of doses), route of administration, and timing of administration (including intervals between doses).
Bacteria may possess one or more antigens: somatic O -antigen (LPS= lipopolysaccharide), capsular K-antigen (polysaccharide or protein), and flagellar H-antigen (protein), exotoxin, etc. (see Fig. 1).1
NOTE: the term “K-antigen” comes from German Kapsule.
¨ Antibodies
Humoral immunity is due to the production of antibodies, a special class of proteins that are soluble in the body fluids. An antibody is produced by a vertebrate host in response to the introduction of an antigen into the body, and it binds specifically with the antigen that stimulated its formation. Antigen-antibody reactions protect the host against many detrimental effects of intruding microbes or other foreign substances.
Antibodies are also called immunoglobulins because they participate in immune reactions and belong to a class of serum proteins called globulins Antibodies are monospecific molecules - they combine only with the single type of antigenic determinant that stimulate their formation. Most antibodies are also bivalent - they possess two identical reactive sites and can couple with two identical antigenic determinants. The reaction between antibody and antigen results in the formation of an antigen-antibody complex.
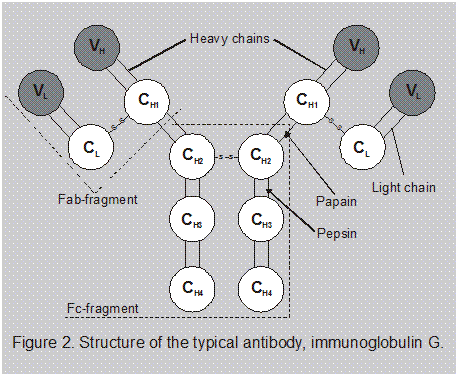 |
• Antibody structure. Although some structural variations exist among antibodies, the typical antibody molecule consists of four protein chains linked together by disulfide bonds in what is usually illustrated as a Y-shaped structure. The two shorter chains, called light (L) chains, are covalently linked to the branches of the longer heavy (H) chains. Each chain has variable and constant regions. Both H and L chains are devided into constant region domains (designated CH and CL) and variableregion domains (designated VH and VL) (Fig. 2). The specificity of the antibody’s combining sites for antigen is determined by the amino acid sequence in the variable regions of both the H and L chains.
The amino acid sequence in the constant region determines other characteristic properties of the antibody, such as its ability to cross chain tissue barriers, to activate the complement system, or to adhere to phagocytic cells. The properties of the tail portion, called the Fc region, define the five major classes of immunoglobulins.
1) Immunoglobulin G (Ig G) is the most common class of antibodies, comprising 80 percent of the antibodies found in the blood. Ig G is typically Y-shaped and bivalent. It crosses the placenta, so that a mother’s Ig G antibodies help to protect her developing fetus. When coupled with antigen, Ig G activates the complement cascade.
2) Immunoglobulin M (Ig M) antibodies consist of five Y-shaped subunits linked together by disulfide bonds in the Fc region. They are the first antibodies to appear after initial exposure to antigen. They fix complement but do not cross the placenta.
3) Immunoglobulin A (Ig A) is a class of antibodies found in two forms. In the serum, Ig A structure resembles that of Ig G. The other form, called secretory Ig A, is the principal antibody found in saliva, mucus, tears, milk, and other external secretions. Secretory Ig A is a dimer composed of two Ig A molecules coupled in the tail region. It is readily secreted across mucous membranes, providing local protection on surfaces of such areas as the alimentary, respiratory, and genitourinary tracts.
4) Immunoglobulin E (Ig E) attaches by its Fc fragment to certain host cells (e.g., mast cells), leaving its antigen-combining sites available for binding with antigen. Ig E is responsible for one group of allergic reactions, the best known of which are hay fever and asthma. Its protective function is detected primarily against parasites.
5) Immunoglobulin D (Ig D) is found in very low concentrations in blood. It is bound to the surface of B lymphocytes and is believed to be necessary for the differentiation of immune cells.
Some properties of the five human immunoglobulin classes are shown in Table 12-1.
Table 12-1